S D P Block Periodic Table

Learn about modern periodic table nomenclature of elements with z 100 s block elements p block elements f block elements and d block elements.
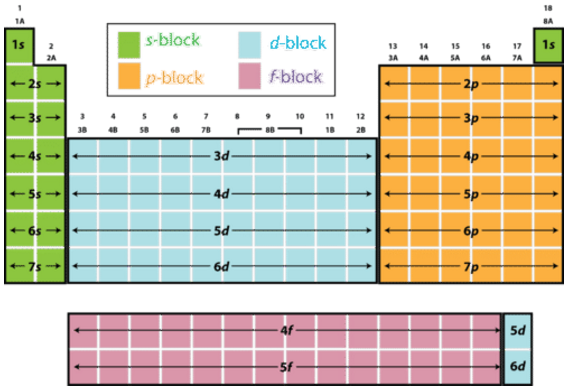
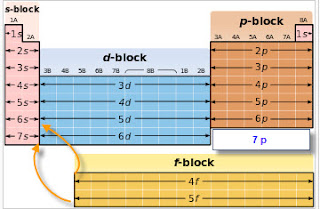
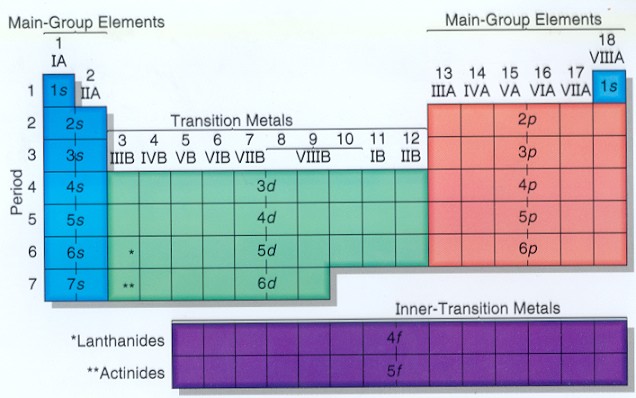
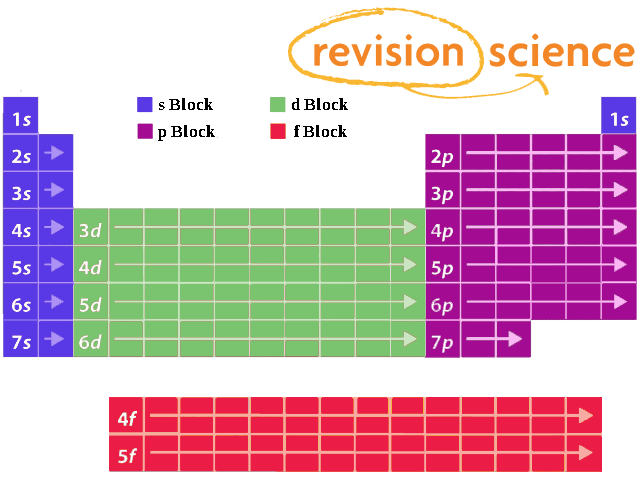
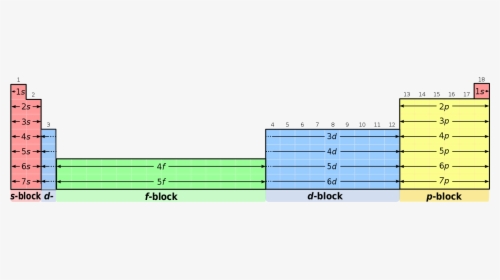
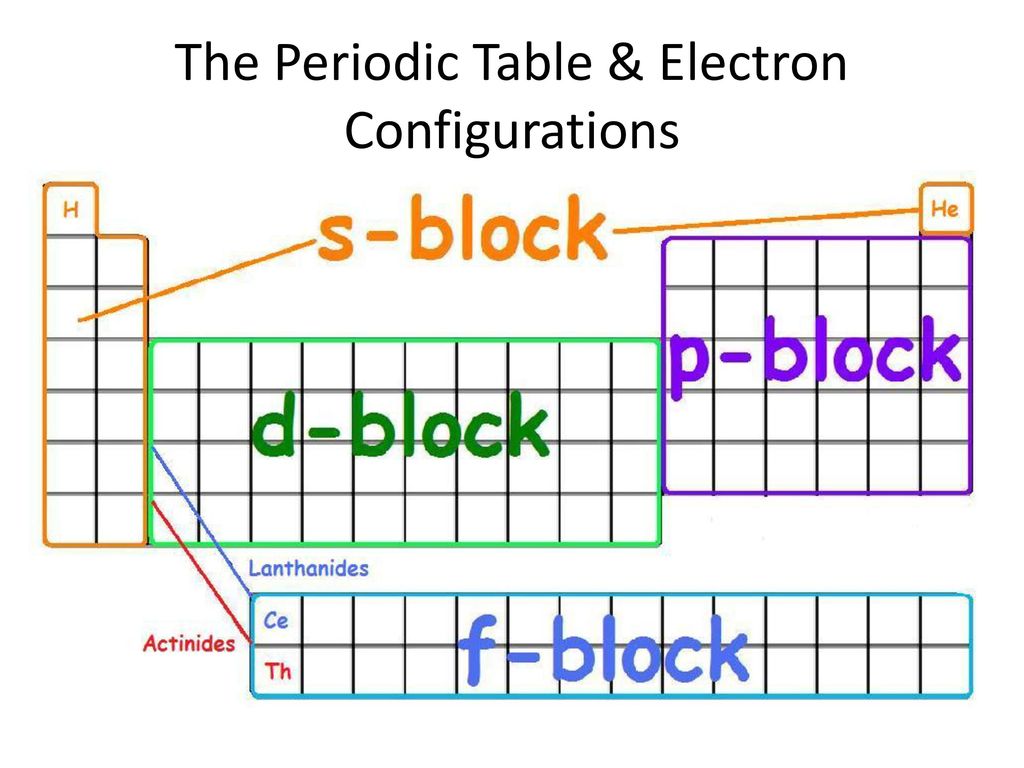
S d p block periodic table. The highest energy level valence shell contains only 1 electron in an s subshell. There are 35 p block elements all of which are in p orbital with valence electrons. The elements in which the last electron enters the s sub shell of their outermost energy level are called s block elements. A block of the periodic table is a set of elements unified by the orbitals their valence electrons or vacancies lie in.
The division of elements into blocks is primarily based upon their electronic configuration as shown in fig. The block names s p d f originated from descriptions of spectroscopic lines of atomic orbitals. What is an element block. These are s p d and f blocks.
Sharp principal diffuse and fundamental. The p block elements are a group of very diverse elements with a wide range of properties. An element block is a set of elements located in adjacent element groups charles janet first applied the term in french. The term appears to have been first used by charles janet.
Each block is named after its characteristic orbital. They constitute groups 3 to 12 in the periodic table. D block elements on modern periodic table. The block names s p d and f are derived from the spectroscopic notation for the value of an electron s azimuthal.
The elements in which the last electron enters the d orbitals of their last but one called penultimate energy level constitute d block elements this block consists of the elements lying between s and p blocks starting from fourth period and onwards. S block p block d block and f block. The labels s p d and f blocks of the periodic table refer to the subshell that is being filled with electrons.